Environmental Atlas – Methodology
The parameters observed the “Environmental Atlas – Methodology” and are to characterize local and regional surface waters quality. Characterization of waters by the “LAWA Method” (Länderarbeitsgemeinschaft Wasser 1991) proceeds on the basis of a variety of parameters and is summarized for a total evaluation. For this work, however, 5 of the parameters most important for eutrophication of Berlin waters were considered, separately evaluated and presented. They are orthophosphoric-phosphorus, ammonium-nitrogen, the oxygen saturation index, oxygen minimum, and Titer for Escherichia coli. A clear and differentiated presentation of the relatively small investigation area of Berlin can thus be made.
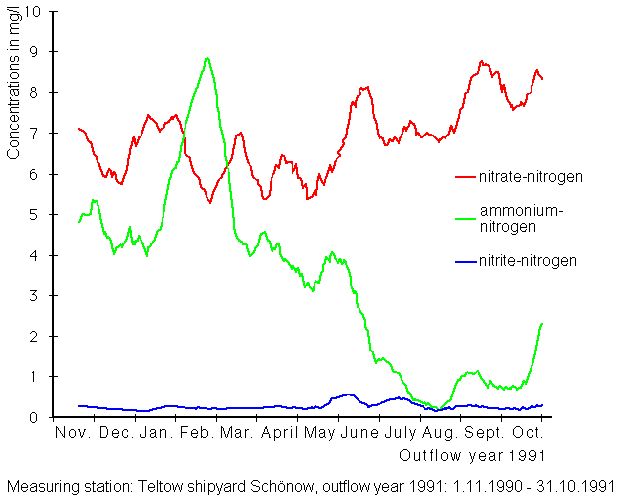
Following the example of water quality maps of the Federal Republic of Germany, classifications were made into 4 quality classes with 3 intermediary levels. Class limits for the 2 oxygen parameters followed water quality mapping classes chosen for use by the LAWA. Concentrations of orthophosphoric-phosphorus and ammonium-nitrogen nutrients are categorized into quality classes so that load levels of the various parameters can be compared. Phosphorus amounts are the limiting factor for algae growth. The eutrophication threshold for dumping up running waters is generally given as 0.01 – 0.03 mg/l. The value 0.01 mg/l is thus the upper limit of quality class 2, “moderately polluted”. The classification for ammonium-nitrogen was taken from the Rhine River report of 1978, in which ammonium-nitrogen was classified into 7 categories (IWAR 1978).
Bacteriological parameters of Escherichi coli (E. coli) are observed here in the presentation of water quality for many waters in Berlin used for swimming and water recreation.
Only the most important running waters in Berlin and some running water sections in the state of Brandenburg directly bordering Berlin are included in the appended map. Waters were divided into 99 sections, each usually with a measuring point in the middle of the section. The study results of these measuring sites are considered representative for the entire section.
Values appearing in the summer half-year (1 May to 31 October) were used in order to measure the time-span of biological activity particularly critical for polluted waters. Parameters observed for orthophosphoric-phosphorus, ammonium-nitrogen, and the oxygen-saturation index were mean values of the summer half-year. The most unfavorable single value in this time span is given for oxygen levels and E. coli Titer.
Measuring results are evaluated and depicted in differentiated colors, according to a 7-step scale from “practically unpolluted” to “extremely polluted”, similar to earlier presentations of other outflow years in the Environmental Atlas.
Orthophosphate-Phosphorus (PO4-P)
Phosphates exist in water in various forms, but phosphates can only be taken up and used by plants to build up their own physical biomass in the form of dissolved orthophosphoric ions.
The majority of phosphates in Berlin waters come from domestic effluents, particularly from feces. The use of cleaning agents containing phosphates also contributes to phosphate loads.
A large portion of Berlin sewage waters is dephosphorized in sewage treatment plants today by biological phosphate elimination or by chemical phosphate removers.